UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a‑16 OR 15d‑16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
FOR THE MONTH OF DECEMBER 2020
COMMISSION FILE NUMBER 001-39081
BioNTech SE
(Translation of registrant’s name into English)
An der Goldgrube 12
D-55131 Mainz
Germany
+49 6131-9084-0
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20‑F or Form 40‑F: Form 20‑F ☒ Form 40‑F ☐
Indicate by check mark if the registrant is submitting the Form 6‑K in paper as permitted by Regulation S‑T Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form 6‑K in paper as permitted by Regulation S‑T Rule 101(b)(7): ☐
DOCUMENTS INCLUDED AS PART OF THIS FORM 6-K
On December 22, 2020, BioNTech SE (the “Company”) held a press conference and video webcast to provide an update on the status of the COVID-19 vaccine development program of its lead vaccine candidate BNT162b2. The presentation materials attached hereto as Exhibit 99.1.
SIGNATURE
Pursuant to the requirements of the Exchange Act, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
BioNTech SE |
|
|
|
|
|
|
|
|
|
|
|
By: |
/s/ Dr. Sierk Poetting |
|
|
|
Name: Dr. Sierk Poetting |
|
|
|
Title: Chief Financial Officer |
Date: December 22, 2020
EXHIBIT INDEX
|
|
|
|
Exhibit |
Description of Exhibit |
|
|
|
|
99.1 |
|
|
|
|
|
|
|

Update on our COVID-19 vaccine December 22, 2020 Exhibit 99.1

This slide presentation includes forward-looking statements Forward-Looking Statements Various statements in this slide presentation concerning the future expectations of BioNTech, its plans and prospects, including the Company’s views with respect to its efforts to combat COVID-19; the collaboration between BioNTech and Pfizer to develop a potential COVID-19 vaccine; its expectations regarding the potential characteristics of BNT162b2 in its Phase 2/3 trial and/or in commercial use based on data observations to date; the expected time point for additional readouts on efficacy data of BNT162b2 in our Phase 2/3 trial; the nature of the clinical data, which is subject to ongoing peer review, regulatory review and market interpretation; the timing for submission of data for, or receipt of, any marketing approval or Emergency Use Authorization; its contemplated shipping and storage plan, including its estimated product shelf life at various temperatures; and the ability of BioNTech to manufacture and supply the quantities of BNT162 to support clinical development and, if approved, market demand, including our production estimates for 2020 and 2021, are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, as amended. Words such as "expects," "plans," "potential," "target," "continue" and variations of these words or similar expressions are intended to identify forward-looking statements. Such statements are based on the current beliefs and assumptions of the management team of BioNTech and on the information currently available to the management team of BioNTech, and are subject to change. The Company will not necessarily inform you of such changes. These forward looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors that could cause the Company’s actual results, performance or achievements to be materially different than any future results, performance or achievements expressed or implied by the forward-looking statements. Actual results may differ materially from those indicated by these forward-looking statements as a result of various important factors, including but are not limited to: our ability to meet the pre-defined endpoints in clinical trials; competition to create a vaccine for COVID-19; the ability to produce comparable clinical or other results, including our stated rate of vaccine effectiveness and safety and tolerability profile observed to date, in the remainder of the trial or in larger, more diverse populations upon commercialization; the ability to effectively scale our productions capabilities; and other potential difficulties. Any forward-looking statements represent the Company’s views only as of today and should not be relied upon as representing its views as of any subsequent date. The Company explicitly disclaims any obligation to update any forward-looking statements. The mRNA vaccine discussed in this slide presentation is an investigational product being developed by BioNTech and its collaborators and are not currently approved by the FDA, EMA or any other regulatory authority.

Safety information Authorized use in the U.S.: The Pfizer-BioNTech COVID-19 Vaccine is authorized for use under an Emergency Use Authorization (EUA) for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 16 years of age and older. Important safety information from U.S. FDA emergency use authorization prescribing information: Do not administer Pfizer-BioNTech COVID-19 Vaccine to individuals with known history of a severe allergic reaction (e.g., anaphylaxis) to any component of the Pfizer-BioNTech COVID-19 Vaccine. Appropriate medical treatment used to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of Pfizer-BioNTech COVID-19 Vaccine. Immunocompromised persons, including individuals receiving immunosuppressant therapy, may have a diminished immune response to the Pfizer-BioNTech COVID-19 Vaccine. The Pfizer-BioNTech COVID-19 Vaccine may not protect all vaccine recipients. In clinical studies, adverse reactions in participants 16 years of age and older included pain at the injection site (84.1%), fatigue (62.9%), headache (55.1%), muscle pain (38.3%), chills (31.9%), joint pain (23.6%), fever (14.2%), injection site swelling (10.5%), injection site redness (9.5%), nausea (1.1%), malaise (0.5%), and lymphadenopathy (0.3%). Severe allergic reactions have been reported following the Pfizer-BioNTech COVID-19 Vaccine during mass vaccination outside of clinical trials. Additional adverse reactions, some of which may be serious, may become apparent with more widespread use of the Pfizer-BioNTech COVID-19 Vaccine. Available data on Pfizer-BioNTech COVID-19 Vaccine administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy. Data are not available to assess the effects of Pfizer-BioNTech COVID-19 Vaccine on the breastfed infant or on milk production/excretion. There are no data available on the interchangeability of the Pfizer-BioNTech COVID-19 Vaccine with other COVID-19 vaccines to complete the vaccination series. Individuals who have received one dose of Pfizer-BioNTech COVID-19 Vaccine should receive a second dose of Pfizer-BioNTech COVID-19 Vaccine to complete the vaccination series. Vaccination providers must report Adverse Events in accordance with the Fact Sheet to VAERS at https://vaers.hhs.gov/reportevent.html or by calling 1-800-822-7967. The reports should include the words “Pfizer-BioNTech COVID-19 Vaccine EUA” in the description section of the report. Vaccination providers should review the Fact Sheet for mandatory requirements and Information to Provide to Vaccine Recipients/Caregivers and the Full EUA Prescribing Information for Requirements and Instructions for Reporting Adverse Events and Vaccine Administration Errors.

Conditional market authorization in 27 European States on 21 December for a COVID-19 vaccine Received Approval for Emergency Use / Temporary Use / Conditional Approval in more than 45 countries worldwide (incl. the EU) COMIRNATY® is our official name of our mRNA vaccine in the EU and Switzerland Received first Conditional Marketing Authorizations worldwide for use in the European Union, Switzerland & Norway

COMIRNATY® – The BioNTech-Pfizer COVID-19 vaccine : COVID-19 + mRNA + Community + Immunity Developed 1,000 potential names in April Recommended up to 3 names (Primary + two backups) Creative rationale

A concerted and large-scale global effort Approved Emergency Use Authorization / Temporary Use Approval Rolling application for emergency use authorization to further countries underway. Conditional Marketing Authorization in the EU and Switzerland1 Vaccination with our COVID-19 vaccine already underway under Emergency Use Authorization/TemporaryUse Approval 1The vaccine is indicated for active immunisation to prevent COVID-19 caused by SARS-CoV-2 virus, in individuals 16 years of age and older. Singapore
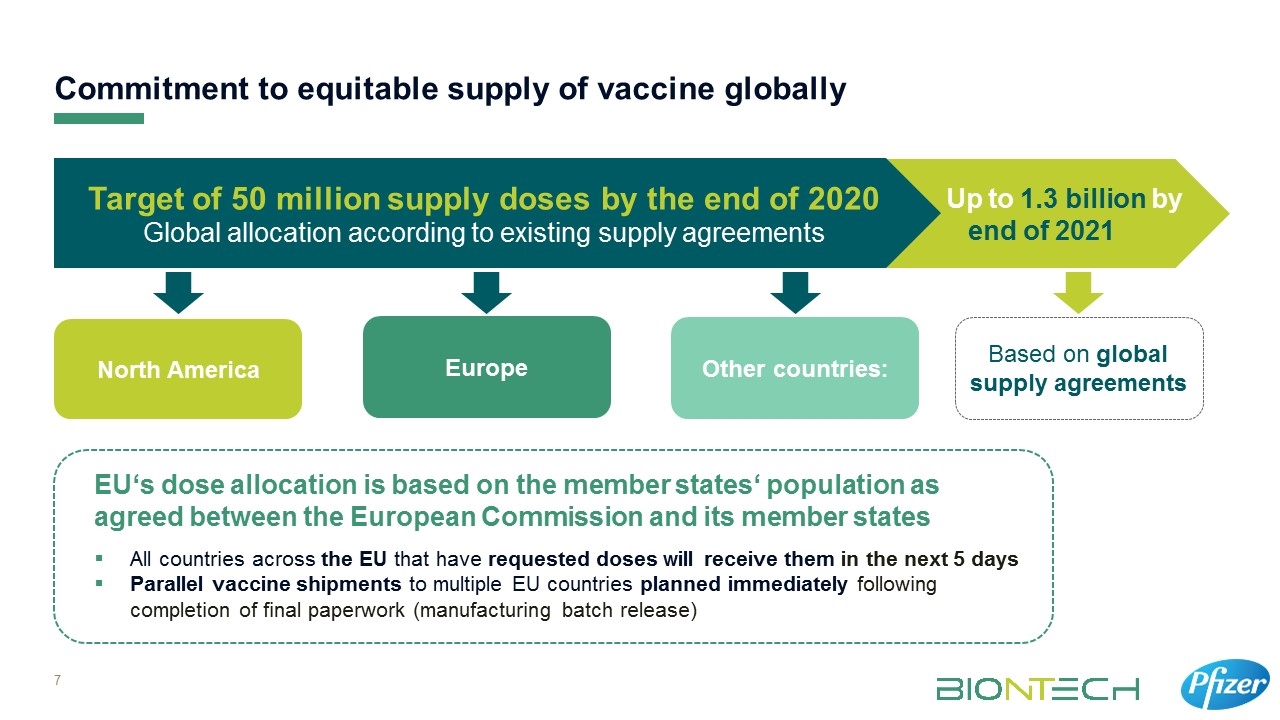
Up to 1.3 billion by end of 2021 Commitment to equitable supply of vaccine globally Target of 50 million supply doses by the end of 2020 Global allocation according to existing supply agreements North America Europe Other countries: EU‘s dose allocation is based on the member states‘ population as agreed between the European Commission and its member states Based on global supply agreements All countries across the EU that have requested doses will receive them in the next 5 days Parallel vaccine shipments to multiple EU countries planned immediately following completion of final paperwork (manufacturing batch release)

Vaccine transportation: minimal changes to pre-existing cold chain Vaccine vials Insulated lid Payload sleeve Outer carton Dry ice pod Pre-packed boxes are transported and distributed to vaccination centers GPS trackers and thermo-sensors relay temperature data to ensure safe delivery At vaccination centers, the vaccines can be stored in delivery boxes and regular fridges Bespoke vaccine freezer boxes; each freezer box can host between approx. 1000 to 5000 doses

Vaccine storage: administered like many other vaccines Administration to vaccinees at room temperature Injected intramuscular (arm); no additional equipment needed for administration at mass vaccination center Once removed from the freezer, the unopened vaccine can be stored for up to 5 days at 2 °C to 8 °C And up to 2 hours at temperatures up to 30 °C, prior to use -70 °C Freezers Long-term storage not necessary at vaccination centers Unless vaccination centers want to store for up to 6 months Special warehouses already identified

Example: decentralized distribution in Germany: BioNTech delivers to 25 distribution centers, run by federal states Accountability of the federal states BioNTech 25 Distribution centers 294 Districts 450 Vaccination centers 100 Mobile vaccination locations delivers to

Example: comprehensive information provided to professionals and vaccination centers in Germany Virtual Service Agent (AI chatbot) BioNTech Service Center Medical Information Center (HCP) Vaccination Center Starter Kit

COMIRNATY®: A journey from scientific discovery to approval

Project Lightspeed – a 10-month journey to an effective and safe vaccine SARS-CoV-2 Genetic Sequence Made Public January 12, 2020 COVID-19 mRNA Vaccine Program Initiation January 27, 2020 Collaborations Fosun Pharma: March 16, 2020 Pfizer: March 17, 2020 Phase 1 / 2 Trial Germany Started April 23, 2020 U.S. Started May 4, 2020 4 vaccine candidates enter clinical testing Initiated Pivotal Phase 2 / 3 Trial July 27, 2020 Lead mRNA vaccine candidate chosen Up to 44,000 subjects FDA Fast Track designation July 13, 2020 Initiated Rolling Submissions EMA: October 6, 2020 Canada: October 7, 2020 UK: October 9, 2020 Singapore New Zealand …and other countries Phase 3 trial meets all primary efficacy endpoints; vaccine efficacy rate of 95% November 18, 2020 Global roll-out has begun Approval for emergency use / temporary supply or Conditional Marketing Authorization in more than 45 countries worldwide including the European Union

Data from Phase 3 study shows 95% efficacy Analysis indicates efficacy rate of 95% in participants with and without prior SARS-CoV-2 infection Final analysis of unblinded data by independent data monitoring committee conducted on Nov 18, 2020 Vaccinated participants will continue to be monitored for efficacy and safety for up to 2 years Vaccinated group Placebo group Number of confirmed COVID-19 cases ≥ day 7 post dose 2 Approx. 44,000 participants Active surveillance for potential COVID-19 symptoms TRIGGERING telehealth or in-person visit and nasal swab Healthy participants 18-85 (> or =16-17,12-15) years of age BNT 162b2 8 cases 162 cases

1 https://www.nejm.org/doi/full/10.1056/NEJMoa2034577?query=featured_home 2 Individuals may not be fully protected until 7 days after their second dose of vaccine. Phase 3 trial data suggest rapid onset of protection against COVID-19 People are vaccinated twice with 30 µg of mRNA, 21 days apart protection against COVID-19 7 days after 2nd dose2 95% Cumulative Symptomatic Incidence of COVID-19 Occurrence1 Days After Dose 1 Onset of protection appears to begin as early as 12 days after the 1st dose Full Immunity

1Subjects without evidence of infection prior to 7 days after dose 2 Clinical data indicate COVID-19 Occurrence From 7 Days After Dose 2 by Comorbidity Status1 BNT162b2 (30 μg) N=18,198 Placebo N=18,325 n Surveillance Time (n) n Surveillance Time (n) VE (%) (95% CI) Overall 8 2.214 (17,411) 162 2.222 (17,511) 95.0 (90.0, 97.9) Comorbidity No comorbidity 4 76 94.7 (85.9, 98.6) Any comorbidity 4 86 95.3 (87.7, 98.8) Any malignancy 1 4 75.7 (-145.8, 99.5) Cardiovascular 0 5 100.0 (-0.8, 100.0) Chronic pulmonary disease 1 14 93.0 (54.1, 99.8) Diabetes 1 19 94.7 (66.8, 99.9) Obese (≥30.0 kg/m2) 3 67 95.4 (86.0, 99.1) Hypertension 2 44 95.4 (82.6, 99.5) Diabetes (including gestational diabetes) 1 20 95.0 (68.7, 99.9)

Clinical trial data indicates vaccine is highly efficacious with a favorable safety profile Gold standard of clinical research – randomized large-scale clinical trial – to ensure safety and efficacy. We took important steps in parallel to accelerate the process together with the authorities – without shortcuts ~44,000 No serious safety concerns Generally well tolerated Clinical Efficacy >94% in all subjects 95% in subjects >65 y/o reported by the independent Data Monitoring Committee (DMC) to date participants in phase 3 trials in U.S., Germany, Turkey, South Africa, Brazil and Argentina More than 40% between 65-85 years of age Observed side-effects are common reactions to vaccination and transient.1 Adverse events were usually mild to moderate in intensity and resolved within a few days after vaccination. 1Full safety assessment has been completed for ~38,000 study participants; BioNTech is also collecting safety data from adolescents and planning a pediatric study and a study on any effects on pregnancy Headache 2.0% Fatigue 3.8% The only Grade 3 adverse events greater than 2% in frequency following dose 2 were: Most frequently observed adverse events were injection site pain and swelling, fatigue, headache, muscle pain, chills, joint pain and fever.

How mRNA works: A deep dive into the technology

What is messenger RNA? The first molecule of life, involved in almost all aspects of cell biology Can be synthesized and engineered to resemble mRNA molecules as they occur naturally in the cytoplasm of human cells and transiently deliver proteins of interest mRNA has a transient messenger function and is rapidly degraded in the body Stable genetic information active for a few days active for a hours to weeks mRNA
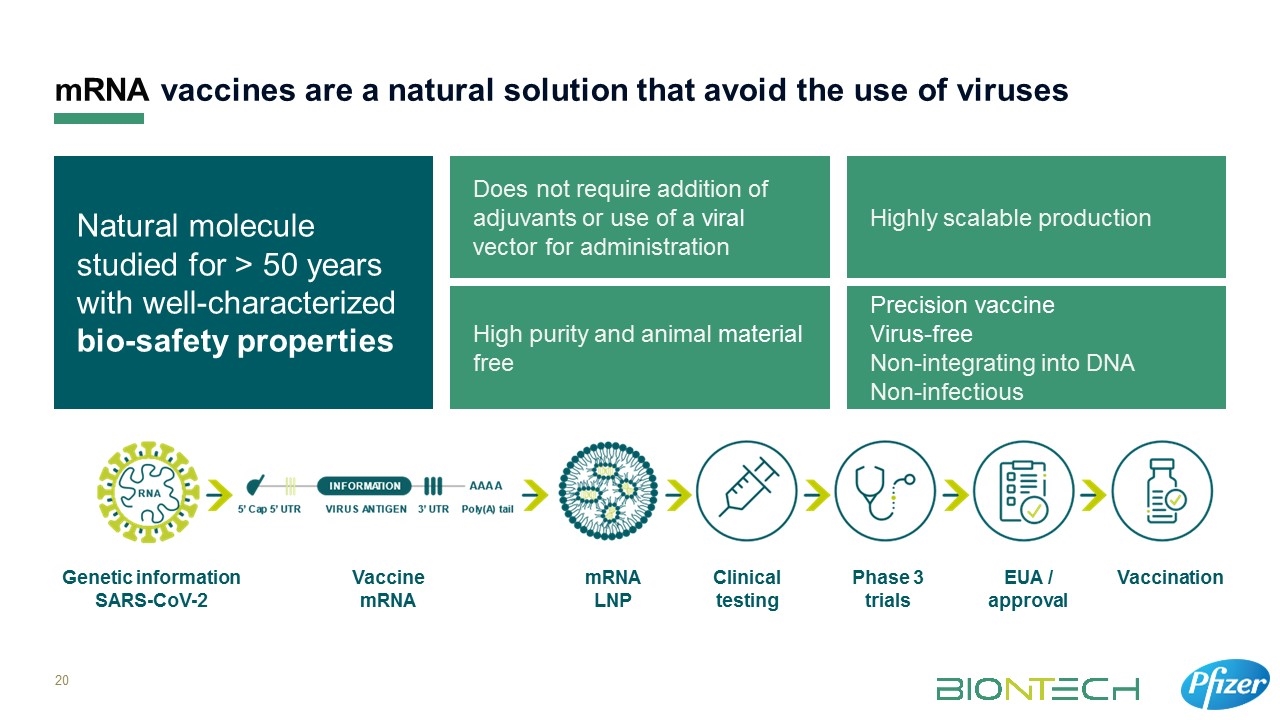
High purity and animal material free Precision vaccine Virus-free Non-integrating into DNA Non-infectious Natural molecule studied for > 50 years with well-characterized bio-safety properties Does not require addition of adjuvants or use of a viral vector for administration Highly scalable production mRNA vaccines are a natural solution that avoid the use of viruses Genetic information SARS-CoV-2 Vaccine mRNA mRNA LNP Clinical testing Phase 3 trials EUA / approval Vaccination

How mRNA vaccines work – training the immune system for a real infection modRNA formulated in LNP enters cell 1 2 mRNA is released 3 Spike protein is made and processed 4 APCs present Spike protein fragments CD4+ Helper T Cell CD8+ Cytotoxic T Cell Eliminates virus infected cells; potentially increases length of protection Activates T and B cells B Cell Virus Neutralizing Antibodies Bind Spike proteins and prevent virus infection of human cells Memory T and B cells Provide immune memory to ensure longer-term protection against SARS-CoV-2 4 AAAA polytail Cap 3’UTR Spike 5’UTR

Multiple levers of immune response: Strong antibody and robust T cell responses observed Immunogenicity*3 Tolerability*4 Antibody Responses*4 T Cell Responses*5 No or only transient viral shedding in SARS-CoV-2 Virus Challenge Local reactions and systemic events mostly mild to moderate and transient in effect Strong SARS-CoV-2 neutralizing antibody responses in both younger and older adults. Antibodies able to neutralize pseudo-viruses representing 19 diverse SARS-CoV-2 variants*6 Strong expansion of multifunctional CD8+ and TH1-type CD4+ T cells. T cell responses directed against multiple regions of the spike protein, including the RBD*6 BioNTech Publications: Holtkamp et al. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 2006. Orlandini von Niessen et al. Improving mRNA-based therapeutic gene delivery by expression-augmenting 3' UTRs identified by cellular library screening. Molecular Therapy, 2019. Vogel et al. A prefusion SARS-CoV-2-spike RNA vaccine is highly immunogenic and prevents lung infection in non-human primates. Nature 2020. Walsh et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates: New England Journal of Medicine, 2020 Sahin et al. Concurrent human antibody and TH1-type cell responses elicited by a Covid-19 RNA vaccine. Nature, 2020. Polack et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine: New England Journal of Medicine, 2020 Sahin et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. Medrxiv, 2020.
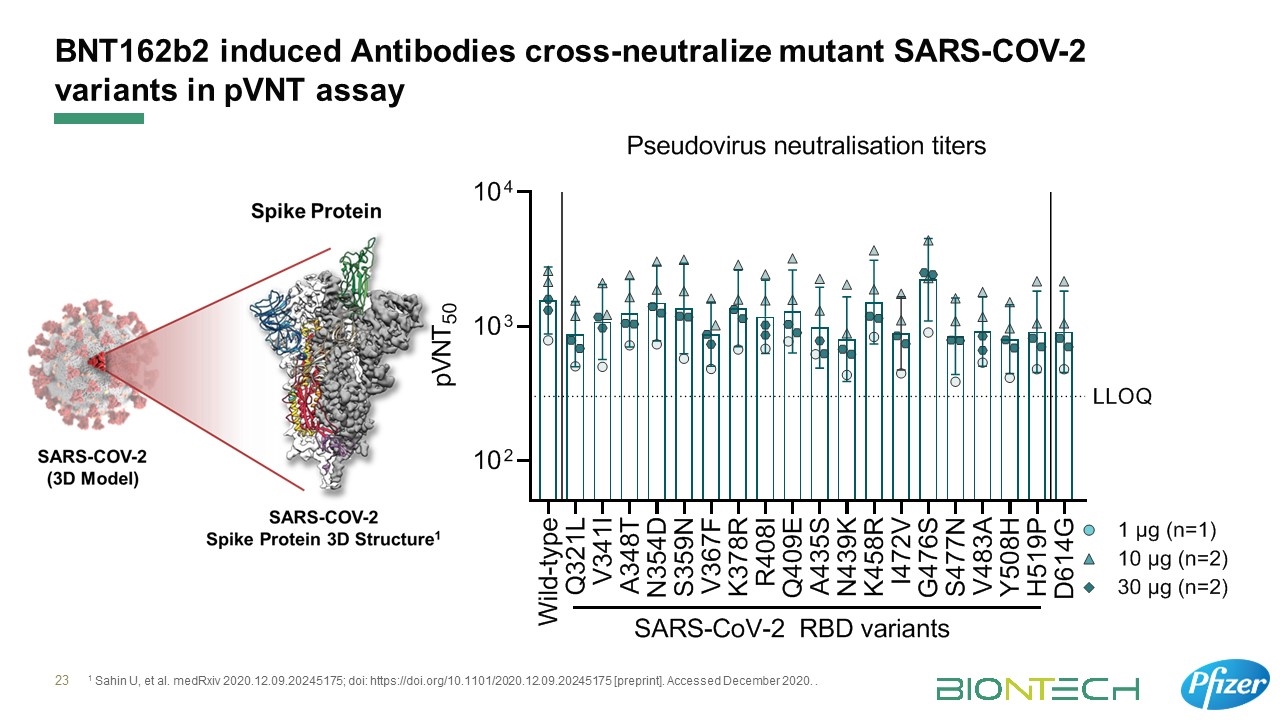
BNT162b2 induced Antibodies cross-neutralize mutant SARS-COV-2 variants in pVNT assay 1 Sahin U, et al. medRxiv 2020.12.09.20245175; doi: https://doi.org/10.1101/2020.12.09.20245175 [preprint]. Accessed December 2020. .

What does COMIRNATY® contain – and why? mRNA* Salt Sugar Lipids 4 different salts Sucrose These buffer the vaccines to stabilize the pH, so that it matches the pH in our bodies. This is a cryoprotectant. It ensures the lipids don’t get too sticky at cold storage temperatures. 4 different molecules Active ingredient They form a protective capsule around the RNA, aiding in the delivery of the RNA, and protect the RNA from degradation. This encodes the viral spike glycoprotein of the SARS-CoV-2 virus. * Each dose: of 0.3 mL with 30 micrograms mRNA Water for injection

What COMIRNATY® contains in detail – from the prescribers information mRNA: Active Ingredient mRNA Salt: 4 different salts potassium chloride potassium dihydrogen phosphate sodium chloride disodium phosphate dihydrate Sugar: Sucrose Sucrose Lipids: 4 different molecules ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate) (ALC-0315) 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide (ALC-0159) 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC) cholesterol + Water for injection

Q&A

An der Goldgrube 12 55131 Mainz Germany T: +49 6131 9084-1074 M: investors@biontech.de

Thank you for your participation! The press conference has now ended.
