UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a‑16 OR 15d‑16 UNDER THE SECURITIES EXCHANGE ACT OF 1934
FOR THE MONTH OF JANUARY 2020
COMMISSION FILE NUMBER 001-39081
BioNTech SE
(Translation of registrant’s name into English)
An der Goldgrube 12 D-55131 Mainz Germany
+49 6131-9084-0
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20‑F or Form 40‑F: Form 20‑F ☒ Form 40‑F ☐
Indicate by check mark if the registrant is submitting the Form 6‑K in paper as permitted by Regulation S‑T Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form 6‑K in paper as permitted by Regulation S‑T Rule 101(b)(7): ☐
EXHIBITS
|
Exhibit |
Description of Exhibit |
|
|
|
|
99.1 |
Presentation: Corporate Update January 2020. |
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
BioNTech SE |
|
|
|
|
|
|
|
|
|
|
|
By: |
/s/ Dr. Sierk Poetting |
|
|
|
Name: Dr. Sierk Poetting |
|
|
|
Title: Chief Financial Officer |
|
|
|
|
Date: January 13, 2020

Corporate Presentation January 2020 Exhibit 99.1

This slide presentation includes forward-looking statements Forward-Looking Statements Various statements in this slide presentation concerning the future expectations of BioNTech, its plans and prospects, including the Company’s views with respect to the potential for mRNA therapeutics, its expectations with respect to the timing and results of clinical trials and release of clinical data (both in respect of its proprietary product candidates and of product candidates of its collaborators), the development of commercial capabilities and the transition of BioNTech to a fully integrated biopharmaceutical company, its expectations with respect to interactions with regulatory authorities such as FDA and EMA, including the potential approval of BioNTech’s or its collaborators’ current or future drug candidates, and expected royalty and milestone payments in connection with BioNTech’s collaborations, constitute forward-looking statements. Words such as "expects," "plans," "potential," "target," "continue" and variations of these words or similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Such statements are based on the current beliefs and assumptions of the management team of BioNTech and on the information currently available to the management team of BioNTech, and are subject to change. The Company will not necessarily inform you of such changes. These forward looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors that could cause the Company’s actual results, performance or achievements to be materially different than any future results, performance or achievements expressed or implied by the forward-looking statements. Actual results may differ materially from those indicated by these forward-looking statements as a result of various important factors, including the initiation, timing, progress, results and cost of the Company's research and development programs and its current and future preclinical studies and clinical trials; the timing of and the Company's ability to obtain and maintain regulatory approval for its product candidates; the Company's ability to identify research opportunities and discover and develop investigational medicines; the Company's expectations regarding the size of the patient populations for its product candidates, if approved for commercial use; the Company's estimates of its expenses, ongoing losses, future revenue and capital requirements and its needs for or ability to obtain additional financing; the Company's ability to identify, recruit and retain key personnel; the Company's and its collaborators' ability to protect and enforce its intellectual property protection for its proprietary and collaborative product candidates, and the scope of such protection; the development of and projections relating to the Company's competitors or its industry; the Company's ability to commercialize its product candidates, if approved; the rate and degree of market acceptance of the Company's investigational medicines; the Company's ability to manage its development and expansion; regulatory developments in the United States and foreign countries; the Company's ability to manufacture its product candidates with advantages in turnaround times or manufacturing cost; and the Company's ability to implement, maintain and improve effective internal controls. The preceding list is not intended to be an exhaustive list of all of the Company's forward-looking statements. Any forward-looking statements represent the Company's views only as of today and should not be relied upon as representing its views as of any subsequent date. The Company explicitly disclaims any obligation to update any forward-looking statements. The mRNA vaccines and other product candidates discussed in this slide presentation are investigational products being developed by BioNTech and its collaborators and are not currently approved by the FDA, EMA or any other regulatory authority.

Agenda Who we are and what we do Our key platforms and programs Outlook in 2020 and beyond +

Our Vision: We aspire to individualize cancer medicine Executive Summary Large addressable market opportunity in solid tumors Targeting some of the largest oncology indications with first-in-class potential Commercialization or co-commercialization rights retained in key geographies World-leading collaborators 7 pharmaceutical collaborators and multiple leading academic institutions 50:50 cost and profit share agreements with leaders in oncology2 Broad & diversified pipeline 10 product candidates in the clinic First registrational trial start in H2 20203 Next generation immunotherapies for cancer and other diseases Vertical integration with in-house manufacturing Over 1,300 employees (~500 in R&D)1 Up to 5 clinical data updates expected in the next 12 months 1As of Dec 31, 2019; 2with Genentech and Genmab; 3BNT111

Our unique approach Harnessing the full potential of the immune system Broadening the universe of eligible patients Improving the treatment success rate Focusing on curative approaches Overview

Achievements 2019 and Outlook 2020 2019 accomplishments: Raised $225m in Series B financing and $149m in Nasdaq IPO Initiated 6 clinical trials across 2 drug classes and 4 different platforms Entered into strategically important agreements with Bill & Melinda Gates Foundation and Regeneron Site for building new iNeST manufacturing facility purchased, planning and design work initiated, European Investment Bank funding secured Goals for 2020: Start 8 or more clinical trials (alone or with our collaborators) Move FixVac into a pivotal phase III trial and iNeST into additional phase II/III clinical trials Further invest in individualized manufacturing capacities Establish presence on East Coast of U.S.

We own in-house manufacturing capabilities for individualized treatments mRNA Manufacturing: Unique process utilizing digitization and automation/robotics to ensure robust, consistent repeatability, quality control and on-demand manufacturing 2 mRNA GMP production facilities: Idar-Oberstein (GMP since 2011) and Mainz (GMP since 2018) Completion and GMP licensure of new Mainz facility for iNeST expected in 2022/23 We intend to further strengthen our position as a leader in the highly automated, on-demand production of individualized therapies. Manufacturing footprint Cell & Gene Therapy Manufacturing: Innovative and robust cell therapy manufacturing process Idar-Oberstein: GMP certified cell and gene therapy facility since 1999 Ongoing facility expansion providing additional, state-of-the-art cell therapy manufacturing capacity

A technology agnostic approach increases our addressable market… Our approach Cancer segment Patient Population Addressed Problem Our Therapeutic Strategy High mutational burden/ adjuvant stage cancers Significant portion of cancer patients Poor risk-benefit profile of checkpoint inhibitors mRNA Neoantigen Immunotherapy (iNeST) Low mutational burden cancers >60% of cancers Poor response to checkpoint inhibitors Shared Antigens (FixVac, CAR-T cells, Antibodies) “Immune desert” cancers >40% of high-mutational cancers Poor infiltration and activation of T-cells into TME mRNA Immunotherapy Immunostimulatory Compounds (intratumoral, RiboCytokines) Cancers with MHC / B2M loss 20-30% of CPI exposed advanced cancers Failure of immune system to recognize tumor cells Antibodies CAR-Ts Refractory tumors Patients with multiple resistance mechanisms Few treatment options Engineered Cell Therapies Combination Therapies We believe a molecular classification and segmentation of cancer types based on an understanding of the challenges of current therapies is best suited to address the challenge of cancer.

…and enables us to exploit our proprietary cancer antigen library Our approach Tumor Associated Antigens (TAAs) Cancer-selective antigens Over the past 15 years, we have built up a database of ~200 of tumor associated antigens, including proprietary targets Viral Neoantigens Mutant Neoantigens Virus-derived proteins Safe and promising targets for immunotherapy: Absent from any non-infected tissue Highly immunogenic Not subject to immune escape Antigens derived from sequence-altered (mutated) proteins Promising targets for cancer immunotherapy: Drive highly specific activation of the immune system (recognized as foreign) Exempt from central tolerance Description and rationale Antigen type and platform Cancer-Germline and Cancer-Embryo-Fetal Antigens FixVac Antibodies CAR-T Tissue Restricted Differentiation Antigens FixVac Antibodies Tumor-Associated Carbohydrate Antigens Antibodies CAR-T Viral Oncoantigen Targets E6 & E7 for FixVac program in HPV16+ H&N cancer FixVac Mutant Neoantigens for individualized Neoantigen Specific Immunotherapy (iNeST) iNeST
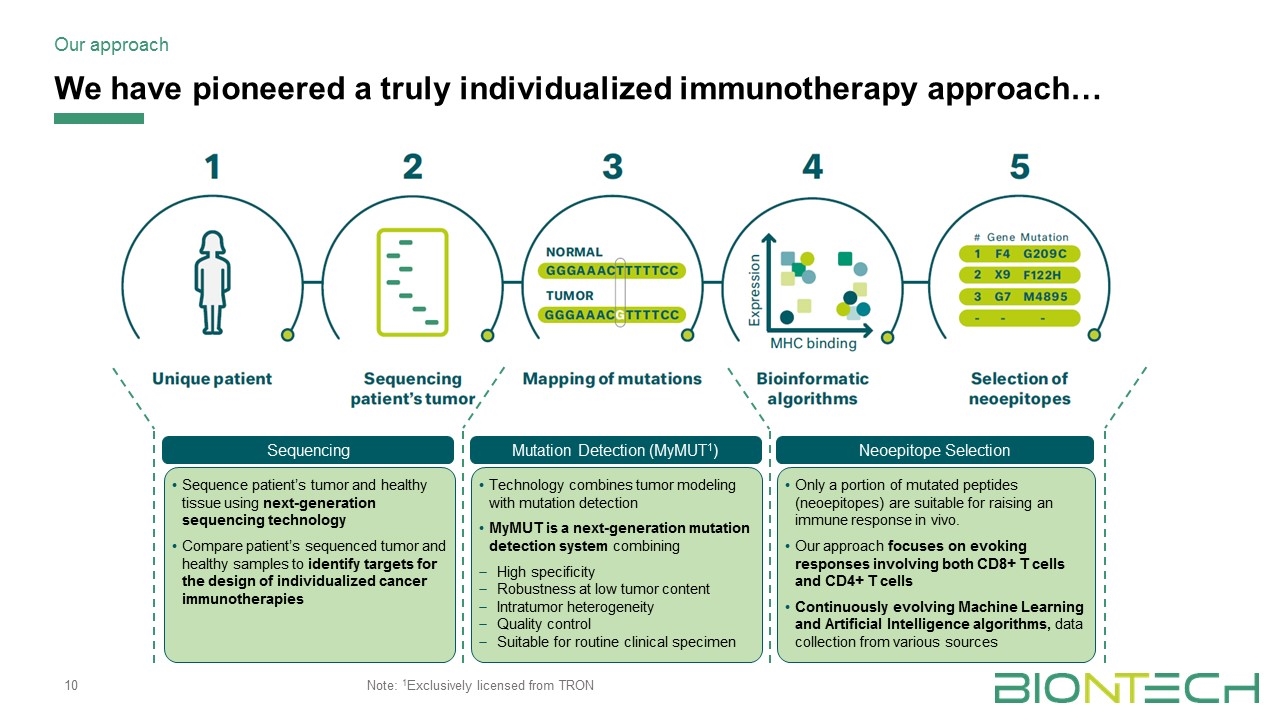
We have pioneered a truly individualized immunotherapy approach… Our approach Sequencing Sequence patient’s tumor and healthy tissue using next-generation sequencing technology Compare patient’s sequenced tumor and healthy samples to identify targets for the design of individualized cancer immunotherapies Mutation Detection (MyMUT1) Technology combines tumor modeling with mutation detection MyMUT is a next-generation mutation detection system combining High specificity Robustness at low tumor content Intratumor heterogeneity Quality control Suitable for routine clinical specimen Neoepitope Selection Only a portion of mutated peptides (neoepitopes) are suitable for raising an immune response in vivo. Our approach focuses on evoking responses involving both CD8+ T cells and CD4+ T cells Continuously evolving Machine Learning and Artificial Intelligence algorithms, data collection from various sources Note: 1Exclusively licensed from TRON

…and ability to leverage deep OMICS capabilities across all our platforms Our approach: deep OMICS capabilities HT NGS HiSeq NovaSeq 6000 10X Genomics Chromium HT qRT-PCR Fluidigm Biomark NGS analysis pipelines seq2HLA MyMut® uMut® Target validation (CD8+, CD4+, antibodies) Pre-clinical models & mode of action Immunology & immune therapies Immune monitoring Flow cytometry and sorting ELISpot Animal models and imaging Syngeneic and xenogeneic models In vivo imaging Target expression RNA vectors Cloning Histology Immunohistochemistry Cryo-immunofluorescence Molecular Cancer Profiling Immune Response Analyses Next-generation sequencing (NGS) Genomics Bioinformatics, Machine Learning, Artificial Intelligence High-Performance Computing Collaboration with TRON Translational Research Center

Collaborating with Leaders in Oncology 50:50 Cost and Profit share (2016) 50:50 Cost and Profit share (2015) Cost and Profit share (2015) Co-development and Co-commercialization of novel mRNA-based, individualized cancer vaccines (iNeST: BNT122) USD 310m upfront & near-term payments 50/50 cost and profit share on global profits Genentech conducting ongoing clinical trials BioNTech with right to co-commercialize in US and certain European markets Co-Development and co-commercialization of Bispecific antibodies (BNT311, BNT312) USD 10m upfront milestones 50/50 cost and profit share on global profits Genmab conducting ongoing clinical trials BioNTech with right to co-commercialize worldwide Development and commercialization of up to 5 intratumoral mRNA cancer immunotherapies, e.g., BNT131 USD 60m upfront and milestones; extended collaboration in 2018 with equity investment Potential for up to EUR 260m in development, regulatory, and commercial milestones on each of the immunotherapies (w/ up to low double-digit royalties on net sales) Option to convert the financial terms for 2 of these immunotherapies to a cost and profit share arrangement (first option exercised) BioNTech with right to co-commercialize in US and certain EU markets Our strategy to commercialize our own products is reflected by differentiated collaboration agreements

Collaborating with Leaders in Oncology, Infectious Diseases and Rare Diseases Our other collaboration agreements are structured to expand our footprint while managing risk Co-development Co-commercialization (2018) Co-development and Co-commercialization agreement for 5 mRNA protein replacement therapies for rare diseases 50/50 global cost and profit share For each co-development project, one or the other party will take lead responsibility for commercialization (and book sales) 5 exclusive oncology LNP licenses to BioNTech – Genevant to receive milestones and royalties on oncology licenses mRNA based prophylactic flu vaccine (BNT161) USD 120m in upfront, equity investment and first milestones Up to USD 325m in potential additional milestone payments Up to very low double-digit royalties on worldwide sales mRNA based vaccines in up to 10 infectious disease collaborations R&D payments to Penn of USD 15m, with USD 5m paid on signing UPenn to conduct preclinical testing of mRNA vaccine compounds BioNTech retains the option to license in the mRNA vaccine candidates for clinical development; milestones and royalties to be paid under certain circumstances HIV and tuberculosis (TB) and up to 3 additional infectious diseases USD 55m as an equity investment to advance prevention and/or treatment of HIV and TB Up to USD 45m in additional grants to fund additional activities in up to 3 additional infectious disease projects within the first 5 years of the collaboration Novel tumor targets and corresponding T-cell receptors USD 60m in upfront and equity investment Potential development, regulatory, and commercial milestones up to an aggregate of approx. USD 300m Up to very low double-digit royalties per drug candidate Licensing Agreement (2018) Strategic R&D Alliance (2018) R&D Agreement (2019) Licensing Agreement (2015) UPenn

We are led by an experienced and entrepreneurial team Management team Supervisory Board Helmut Jeggle Managing Director, Athos Former Head of Business Planning & Analyses at Hexal Michael Motschmann Founder of MIG Verwaltungs AG Significant experience in building companies Prof. Christoph Huber, MD Co-founder of BioNTech Prof. Emeritus at the Mainz University Dr. Ulrich Wandschneider Former CEO at Asklepios Kliniken Scientific Advisory Board Prof. Dr. Rolf Zinkernagel Nobel Prize in Physiology or Medicine in 1996 for his discovery of immune recognition of virus-infected cells Professor Emeritus at Zurich University Prof. Dr. Hans Hengartner Professor Emeritus at ETH Zurich and University of Zurich World renowned immunologist Management Prof. Ugur Sahin, MD Co-Founder and CEO Sean Marett CBO / CCO Dr. Özlem Türeci Co-Founder and CMO Dr. Sierk Poetting CFO / COO Ryan Richardson Chief Strategy Officer

Building a global biotechnology company Company footprint BNT office planned San Diego Mainz Berlin Munich Idar-Oberstein USA Germany

Agenda Who we are and what we do Our key platforms and programs Outlook in 2020 and beyond +

We have a broad pipeline of mRNA product candidates in oncology Our pipeline Drug Class Platform Product Candidate Indication (Targets) Preclinical Phase 1 Phase 2 Rights Collaborator Milestones Oncology mRNA FixVac (fixed combination of shared cancer antigens) BNT111 advanced melanoma (adjuvant & metastatic) fully-owned report phase 1 data and phase 2 start 1H 2020; phase 3 start 2H 2020 BNT112 prostate cancer fully-owned first patient enrolled in phase 1/2 in Dec 2019 (plan: 2H 2019) BNT113 HPV+ head and neck cancer1 fully-owned phase 2 start 2H 2020 BNT114 triple negative breast cancer fully-owned data update 1H 2020 BNT115 ovarian cancer1 fully-owned BNT116 NSCLC fully-owned - iNeST (patient specific cancer antigen therapy) RO7198457 (BNT122) 1L melanoma with CPI2 Genentech (global 50:50 profit/loss top line data 2H 20203 multiple solid tumors data update 2020 Intratumoral Immunotherapy SAR441000 (BNT131) solid tumors (IL-12sc, IL-15sushi, GM-CSF, IFNα) Sanofi (global profit/ loss share) data update 2H 20204 1BNT113 and BNT115 are currently being studied in an investigator-initiated Phase 1 trial; 2Checkpoint Inhibitor; 3We expect this topline data update to include an update on the ongoing study, including patient enrollment numbers, with full efficacy and safety data for an interim update expected in the second half of 2021; 4As the trial is sponsored and conducted by Sanofi, the timing of data updates is not under our control, and is subject to change by Sanofi new new

We have a broad pipeline of mRNA product candidates in oncology... Our pipeline Drug Class Platform Product Candidate Indication (Targets) Preclinical Phase 1 Phase 2 Rights Collaborator Milestones Oncology mRNA RiboMabs (mRNA-encoded antibodies) BNT141 multiple solid tumors fully-owned phase 1 start 2H 2020 BNT142 multiple solid tumors (CD3+CLDN6) fully-owned phase 1 start 1H 2021 RiboCytokines (mRNA-encoded Cytokines) BNT151 multiple solid tumors (optimized IL-2) fully-owned phase 1 start 1H 2020 BNT152+ BNT153 multiple solid tumors (IL-7, IL-2) fully-owned phase 1 start 1H 2021

We expect additional oncology trial starts in 2020 - with first data in 2021 Our pipeline Drug Class Platform Product Candidate Indication (Targets) Preclinical Phase 1 Phase 2 Rights Collaborator Milestones Oncology Engineered Cell Therapies CAR-T Cells BNT211 multiple solid tumors (CLDN6) fully-owned phase 1/2 start 1H 2020 BNT212 pancreatic, other cancers (CLDN18.2) fully-owned - TCRs Undisclosed undisclosed Eli Lilly (exclusive license) - To be selected all tumors fully-owned - Antibodies Next-Gen CP5 Immunomodulators GEN1046 (BNT311) multiple solid tumors (PD-L1×4-1BB) Genmab (global 50:50 profit/loss) data update 1H 2021 GEN1042 (BNT312) multiple solid tumors (CD40×4-1BB) - Targeted Cancer Antibodies BNT321 (MVT-5873) pancreatic cancer (sLea) fully-owned patient enrolled to resume phase 1 in Dec 2019 (plan: 2H 2019) SMIM6 Toll-Like Receptor Binding BNT411 solid tumors (TLR7) fully-owned phase 1 start 1H 2020 5Checkpoint; 6Small Molecule Immunomodulators new

Our first trial starts outside of oncology are expected by the end of 2020 Our pipeline Drug Class Platform Indication (Targets) Discovery Preclinical Phase 1 Phase 2 Rights Partner Milestones Other mRNA Infectious Disease Immunotherapies Influenza Pfizer start first study by end of 2020 up to 10 indications Penn1 first phase 1 trial to start 1H 2021 HIV and tuberculosis Bill & Melinda Gates Foundation Rare Disease PRT2 5 rare disease indications Genevant (global 50:50 profit/loss) first phase 1 trial to start 2H 2020 1We are eligible to receive worldwide licenses; 2Protein Replacement Therapy

Targeting Cancer Immunomodulation mRNA Cancer Vaccines Antibody Targeting Small Molecule Immunomodulators Next Generation Immunomodulators FixVac, iNeST Targeted Antibodies RiboMabs Engineered Cell Therapies CAR-T, TCRs Bispecific Antibodies (CPI blockade + co-stimulation) TLR agonist Engineered Cytokines Intra-tumoral cytokines RiboCytokines Our IO strategy exploits complementary therapeutic platforms A multi-platform approach + We expect to have all core platforms in the clinic by the end of 2020 1 2020 In the clinic 1 1 1 1 1 2020

We own one of the broadest mRNA toolkits in the industry Local Intratumoral Systemic Tissue specific Adjustable activity in vivo from minutes up to weeks Various delivery routes Liposomes / LPX LNPs Polyplexes Broad formulation spectrum Multiple mRNA formats uRNA modRNA saRNA taRNA 5’ Cap 5’ UTR ORF 3’ UTR Poly(A) tail mRNA drug class | RiboMab platform

We have developed multiple proprietary mRNA formats and formulations Our mRNA formats Lipoplex: Our lipoplex formulation, or LPX, embeds the mRNA between a lipid bilayer, which is used for our FixVac and iNeST platforms LNPs: For other applications, we encapsulate our mRNA in lipid nanoparticles, or LNPs. These formulations are suitable for our RiboMab, RiboCytokine and rare disease protein replacement therapy platforms Polyplexes: Our portfolio also comprises polyplexes, which are being utilized in certain of our discovery programs, in which the mRNA is bound to a polymer and then forms nanoparticles Our mRNA delivery formulations Lipoplexes (FixVac, iNeST, CARVac) LNPs (RiboMabs, RiboCytokines, Rare Disease) Polyplexes (Discovery Programs) OUR mRNA FORMATS Uridine mRNA (uRNA) Repeat administration Strong T cell responses Nucleoside-modified mRNA (modRNA) Non-immunogenic vector Strong antibody responses Therapeutic protein delivery Self-amplifying mRNA (saRNA) Sustained expression T cell responses upon prime only Protection upon prime only Trans-amplifying mRNA (taRNA): Replicase and mRNA Sustained expression Highly flexible co-transfer Low antigen RNA doses mRNA drug class | formulations and formats

We are developing multiple mRNA therapeutic platforms mRNA Platform Drug Targets mRNA Formats Delivery Formulations 7 mRNA platforms Broad range of biological targets 4 types of mRNA Multiple optimized formulations Oncology FixVac Shared Antigens uRNA RNA-LPX iNeST Neoepitopes uRNA RNA-LPX Intratumoral Immunotherapy Immunomodulators modRNA Various formulations Intratumoral RiboMabs mAb targets modRNA LNPs Intravenous delivery RiboCytokines Cytokines modRNA Various LNP formulations Other Infectious Disease Vaccines Pathogens saRNA, taRNA, modRNA Various LNPs for i.m. & s.c. delivery Rare Disease Protein Replacement Therapy Diverse Proteins modRNA Liver targeted LNPs uRNA: uridine mRNA; modRNA: nucleoside-modified mRNA; saRNA: self-amplifying mRNA; taRNA: trans-amplifying mRNA; mRNA drug class

Agenda Who we are and what we do Our key platforms and programs FixVac: off-the-shelf mRNA immunotherapy Individualized Neoantigen Specific Immunotherapy Antibody programs Leveraging platform synergies: CARVac + CAR-T Outlook in 2020 and beyond Small molecule immunomodulator program

FixVac: mRNA immunotherapy targeting shared tumor associated antigens Off-the-shelf mRNA immunotherapy Targeting a fixed combination of tumor shared antigens Antigen selection on the basis of antigens shared among patients within a particular cancer type Applicable for almost all types of tumor antigens Proprietary RNA-LPX delivery formulation Allows systemic dendritic cell targeting Strong immunogenicity observed in vivo TLR7-driven adjuvant effect Type-I interferon driven innate and adaptive immune stimulation Overcomes tolerance against self-antigens mRNA drug class | FixVac platform

Cumulative patient coverage of FixVac melanoma targets is over 90% Computational pipeline for antigen-discovery and RT-PCR validation RNA-Seq data from 337 melanoma samples in TCGA Target-criteria High expression in melanoma No expression in toxicity-relevant normal cells and tissues Coverage of as many patients as possible with at least 1 antigen Coverage of a substantial fraction of patients with more than 1 antigen Tyrosinase MAGEA3 NY-ESO-1 TPTE mRNA drug class | FixVac platform

BNT111 has demonstrated strong antigen specific immune responses in melanoma Shared Antigens Targeted NY-ESO-1 / MAGE-A3 / Tyrosinase / TPTE Phase 1 Study Overview Dose escalation to evaluate safety and tolerability 8 treatment cycles Planned total 115 patients with stage III/IV melanoma First patient in: May 27, 2015 95 dosed as of July 29, 2019 Interim Safety Data as of July 29, 2019 The overall adverse event profile was dominated by mild-to-moderate, transient and manageable flu-like symptoms. These symptoms were managed by pre-medication with non-steroidal antipyretics, such as ibuprofen and acetaminophen. Eight of the 95 subjects dosed with BNT111 experienced related treatment-emergent serious adverse events, or TESAEs. There were confounding factors, such as treatment with other therapies or underlying medical conditions, for the subjects with related TESAEs. Interim Immunogenicity Data Immunogenicity analyzed by independent assays including Elispot, intracellular cytokine staining and HLA multimer analyses Subset of 18 patients with IVS Elispot demonstrating de novo and augmented T cell responses in all patients Ex-vivo T-cell responses observed in > 75% of patients Both CD8+ and CD4+ T-cells induced (Th1 Phenotype, PD1+) T-cell responses persisted for months after stop of vaccination mRNA drug class | FixVac platform | BNT111 (FixVac advanced melanoma) Example ex vivo HLA-peptide analysis

FixVac: Induced immune responses were robust Control CMVpp65 HLA-A201 NY-ESO-1 HLA-Cw0304 Pre-Treatment Post-Treatment Objective responses are associated with strong T-cell expansion 1,000 fold expansion within 4-8 weeks Evidence of strong tumor cell killing activity Patient Case: Antigen specific T-cells in blood before/after vaccination mRNA drug class | FixVac platform

FixVac: BNT111 Interim clinical activity data (dose range 14µg -100µg) Summary Advanced melanoma patients (Stage III, IV) Out of 74 patients with available follow-u radiological imaging 42 patients were assessed for preliminary analysis as of July 29, 2019 25 patients with metastatic melanoma who received BNT111 monotherapy following progression on CPI* and in some cases other therapies 3 patients with partial response (PR) 1 patient metabolic complete response 7 patents with stable disease (SD) 14 progressive disease (PD) 17 patients with metastatic melanoma who received BNT111 in combination with CPI after progression on CPI monotherapy 6 patients with partial response (PR) 2 patents with stable disease (SD) 9 progressive disease (PD) Adjuvant cohort of 32 patients still in study mRNA drug class | FixVac platform | BNT111 (FixVac advanced melanoma) *CPI: Checkpoint inhibitor

BNT112: FixVac Prostate Cancer mRNA drug class | FixVac platform | BNT112 (FixVac prostate cancer) Ph1/2: first patient enrolled in December 2019 Multipronged vaccine: Targeted antigens of BNT112 are 5 prostate cancer specific antigens (PAP, PSA and 3 undisclosed antigens) RNA-LPX vaccine format validated by our FixVac Melanoma program Eligible are patients with mCRPC symptomatic patient population after two lines of systemic chemotherapy for treatment with BNT112 alone or in combination with cemiplimab (aPD1, Regeneron) Newly diagnosed high risk localized prostate cancer for treatment with BNT112 in combination with goserelin acetate & cemiplimab (aPD1, Regeneron) followed by surgery Antigen 1 PSA PAP Antigen 4 Antigen 5 Antigen 1 PSA PAP Antigen 4 Antigen 5

FixVac: A flexible format which can rapidly be adapted for different tumors Initiation of additional clinical studies in other solid tumor indications Metastatic melanoma HPV positive head & neck cancer (IIT) Triple negative breast cancer Prostate cancer BNT111 BNT113 BNT114 BNT112 BNT115 Preclinical Phase 1 Phase 2 Product candidate Ph. 2 start H1 2020 Ph. 3 start H2 2020 Ph. 2 start 2H 2020 Data update 1H 2020 First patient enrolled in 2H 2020 5 programs in human trials BNT112 phase 1 trial started in 2019 mRNA drug class | FixVac platform new NSCLC BNT116 Ovarian cancer (IIT)

Agenda Who we are and what we do Our key platforms and programs FixVac: off-the-shelf mRNA immunotherapy Individualized Neoantigen Specific Immunotherapy Antibody programs Leveraging platform synergies: CARVAC + CAR-T Outlook in 2020 and beyond Small molecule immunomodulator program

Conclusions from iNeST clinical trials iNeST Clinical trials Preliminary observations in ongoing trials with BNT122 (IV administration): iNeST can be manufactured for individual patients with clinically relevant turn-around times across a range of tumor types iNeST +/- anti-PDL1 has a manageable safety profile Strong iNeST immunogenicity across a range of tumour types Long-term follow-up of completed trial with BNT121 (Intra-nodal administration): Long-term relapse free disease activity with BNT121 iNeST in adjuvant Melanoma Clinical efficacy evaluation in randomized phase II studies initiated

Update for BNT121 (as of October 2019) iNeST Clinical trials Metastatic Relapse Analyses 9 of 13 patients without documented PFS Events Melanoma Stage IIIb, IIIc, and IV, 13 patients, intranodal delivery against 10 neoantigens Stable progression free survival in adjuvant melanoma

BNT121: Interim clinical activity data (dose range 14µg -100µg) New Metastases Relapse free disease control 13 High relapse risk melanoma patients (Stage IIIb, IIIc, IV) 13 patients 8 5 Stable Disease Complete Response 1 Partial Response 1 Complete Response (Combination with CPI) 1 Progression after temporary disease control (†18 months) 1 Lost to follow-up at 15 months 1 Clinical outcome in follow up Clinical status iNeST | BNT121 8 Neo-epitope RNA production Neo-epitope RNA vaccination, continued treatment, follow up RNA vaccination Metastatic melanoma (N=13) First-in-human Phase 1 with 13 patients with melanoma stage IIIb, IIIc, and IV; intranodal delivery Immune responses against at least one neoantigen in all patients Cumulative rate of metastatic events significantly reduced, resulting in a sustained PFS 3 out of 5 pts with melanoma relapses developed treatment-related objective clinical responses One complete response (CR), relapse-free 26 mon One immunotherapy-related partial response (PR) One CR in combination with anti-PD1 8 patients (no detectable lesions at start) relapse free and recurrence-free for the whole follow-up (12 to 23 months) Sahin et. al. Nature 2017

iNeST: Results expected for phase 1 in 2020, for phase 2 in 2H 2020 Phase 1a/1b in Multiple Solid Tumors: Open-label, dose-escalation study of safety and pharmacokinetics Phase 2 in Advanced Melanoma: Interventional open-label, multicenter randomized study of efficacy and safety Enrollment: Up to 770 Start date: Dec 2017 Data update: 2020 Tumor types:Melanoma, NSCLC, Bladder, CRC, TNBC, Renal, H&N, other solid tumors Phase 1a:Single-Agent Phase 1b:Combination with atezolizumab Primary outcome measures in iNeST + atezolizumab treated participants compared with iNeST-only participants include: Dose-limiting toxicities (DLTs) Adverse Events (AEs) Single-Agent Escalation Combo Escalation (PCV + atezolizumab) Combo Exploration/Expansion Indication-specific expansions, emphasis on detecting combo signal as quickly as possible Enrollment: 132 Start date: Jan 2019 Topline data: 2H 2020 Tumor types:Advanced melanoma Phase 2:Combination with pembrolizumab Study to Evaluate the Efficacy and Safety of iNeST in combination with pembrolizumab vs. pembrolizumab alone in participants previously untreated in advanced melanoma (first-line) Primary endpoint in iNeST+ pembrolizumab treated participants compared with pembrolizumab-only participants: Progression-free Survival (PFS) iNeST | BNT122

Individualized Neoantigen Specific Immunotherapy (iNeST) iNeST summary Overview Dosed first-in-human individualized mRNA immunotherapy Targeting multiple neoantigens Intended to be a universal approach applicable for the majority of cancers 50:50 profit/loss share with Genentech Turnaround time reduced from three months to six weeks BNT121 (i.n.) BNT122 (IV) Preclinical Phase 1 Phase 2 Up to 41 mon follow-up data Data update 2020 Top line data 2H 2020 New planned trial starts in 2020 Multiple solid tumors Metastatic melanoma (N=13) First-line advanced melanoma in combination with pembrolizumab First-line adjuvant solid cancer in combination with Tecentriq First-line adjuvant solid cancer Currently being evaluated in >8 solid tumor indications

Agenda Who we are and what we do Our key platforms and programs FixVac: off-the-shelf mRNA immunotherapy Individualized Neoantigen Specific Immunotherapy Antibody programs Leveraging platform synergies: CARVac + CAR-T Outlook in 2020 and beyond Small molecule immunomodulator program

BNT311 (GEN1046) BNT312 (GEN1042) Next-Gen checkpoint immunomodulators PD-L1 4-1BB K409R F405L Fc-silencing mutations matched CH3 mutations L234F L235E D265A CD-40 4-1BB K409R F405L Fc-silencing mutations matched CH3 mutations L234F L235E D265A Two bispecific antibodies partnered with Genmab Potential “first-in-class” bispecific antibodies Conditional activation of immuno-stimulatory checkpoint activity 50:50 profit/loss share Both programs are now in the clinic Preclinical Phase 1 Phase 2 PD-L1x4-1BB Product Candidate CD-40x4-1BB Ph1/2a Ph1/2a Data update 1H 2021 Antibodies drug class | bispecific antibodies

Characteristics Bi-specific antibody combining constitutive CPI blockade and conditional co-stimulatory activity Enhanced prolife-ration of antigen specific activated T cells in the presence of PD-L1+ cell Constitutive PD-L1 blockade & Conditional 4-1BB agonism 1 2 Increased tumor infiltrating lymphocyte (TIL) expansion in human tumor tissue cultures ex vivo 3 Induced tumor regression of murine tumors superior to pure PD-L1 blockage associated with an increase in tumor-specific CD8 T-cells Mode of Action Antibodies drug class | bispecific antibodies | Anti-PDL1, anti-4-1BB Preclinical antitumor activity beyond PDL1 blockade PDL1 Blockade 41BB Agonism *SITC 2018, Altintas et al Next-Gen checkpoint immunomodulators

Bispecific antibody GEN1046 (BNT311): Phase 1/2a in solid tumors First-in-human, open-label, dose-escalation trial with expansion cohorts to evaluate safety of GEN1046 (PD-L1x4-1BB) in subjects with malignant solid tumors Enrollment: 192 Data update: 1H 2021 Tumor types:Malignant Solid Tumors Intervention: GEN1046 (BNT311) is a PD-L1x4-1BB bispecific antibody that induces conditional activation of T cells through 4-1BB stimulation which is dependent on simultaneous binding to PD-L1 GEN1046 (BNT311) IV once every 21 days Dose levels determined by the starting dose and the escalation steps taken in the trial Description: Open-label safety trial Two parts, a dose escalation (phase 1, first-in-human) and an expansion part (phase 2a) Key Primary endpoints: Dose limiting toxicity Adverse Events Safety laboratory parameters Antibodies drug class | bispecific antibodies | Anti-PDL1, anti-4-1BB

BNT321 (MVT-5873) BNT321: Cancer antibody targeting Cancer Associated Carbohydrate sLea sLea Phase 1 resumed in 2H 2019 Antibodies drug class | targeted cancer antibodies Pancreatic ductal adenocarcinoma Colon carcinoma Lung adenocarcinoma Urinary bladder, mucinous adenocarcinoma Colon metastatic to ovary Breast carcinoma, lymph node AACR 2016, Abstract CT026, Ragupathi_Maffuid sLea expression in human cancers Characteristics MVT-5873 (5B1) was acquired as clinical stage product from MabVax Therapeutics Holdings Inc. in 2019 Fully human IgG1 mAb with subnanomolar affinity, potent cell killing by ADCC &CDC activity Targets sialyl Lewis A epitope (sLea) epitope present in a range of glyco-proteins collectively known as CA19-9. CA19-9 is specifically expressed in pancreatic and various other cancers. Shedded CA19-9 is a prognostic marker in these cancers. CA19-9 is functionally associated with carcinogenesis1. Six patients evaluated in combination with chemotherapy; four of them met the criteria for partial response and two patients met the criteria for stable disease. BNT321 was generally well tolerated by all six patients. First patient enrolled to resume the BNT321 trial against pancreatic cancer in December 2019. 1Engle et al,. Science 2019: The glycan CA19-9 promotes pancreatitis and pancreatic cancer in mice new

Agenda Who we are and what we do Our key platforms and programs FixVac: off-the-shelf mRNA immunotherapy Individualized Neoantigen Specific Immunotherapy Antibody programs Leveraging platform synergies: CARVac + CAR-T Why invest in BioNTech Small molecule immunomodulator program

Complete eradication of advanced tumors in an ovarian carcinoma xenograft model BNT211: Next generation CAR-T targeting CLDN6 with CARVac “primer” Engineered Cell Therapies | CAR-T therapies | BNT211 CLDN6 is not present in healthy tissues CLDN6 is expressed in multiple cancers Ovarian cancer Testicular tumor Lung cancer

Second-generation CAR-T therapy targeting CLDN6 combined with CLDN6 Engineered Cell Therapies | CAR-T therapies | BNT211 1° RNA(LIP) vacc. 2° RNA(LIP) vacc. 3° RNA(LIP) vacc. CLDN6 Ctrl CLDN6 Ctrl CLDN6 Ctrl CART cells (i.v.) d0 d7 d8 d11 d15 d17 d25 d22 ACT RNA-lipoplex vaccine enhances expansion & persistence

Agenda Who we are and what we do Our key platforms and programs FixVac: off-the-shelf mRNA immunotherapy Individualized Neoantigen Specific Immunotherapy Antibody programs Leveraging platform synergies: CARVac + CAR-T Outlook in 2020 and beyond Small molecule immunomodulator program

BNT411: TLR7 agonist has entered the clinical stage Intravenously administered small molecule TLR7 (toll-like receptor 7) agonist Engineered for high potency and high selectivity for TLR7 receptor at the therapeutically active dose range Activates both adaptive and innate immune system Type 1 interferon-dominated release of cytokines and chemokines and potent stimulation of antigen-specific CD8+ T cells, B cells and innate immune cells such as NK cells and macrophages To be used in combination with chemotherapy and checkpoint inhibitors. Qualifies for various solid tumor indications and small cell lung cancer IND was filed on November 5, 2019 We expect to initiate a Phase 1/2a clinical trial as a mono and combination therapy in solid tumors in H1/2020 Small molecule immunomodulator | TLR7 agonist | BNT411 Planned study design for FIH trial: Phase 1/2a, first-in-human, open-label, dose-escalation trial with expansion cohorts to evaluate safety, pharmacokinetics, pharmacodynamics, and preliminary efficacy of BNT411 as a monotherapy in patients with solid tumors and in combination with atezolizumab, carboplatin and etoposide in patients with chemotherapy-naïve extensive-stage small cell lung cancer (ES-SCLC) H1 2020

Agenda Who we are and what we do Our key platforms and programs Outlook in 2020 and beyond +

We expect a significant news flow in the upcoming next 12-18 months Platform Candidate Indication (Target) 1H-2020 2H-2020 20213 20223 FixVac BNT111 Advanced Melanoma BNT112 Prostate Cancer BNT113 HPV+ H&N Cancer BNT114 Triple Negative Breast Cancer iNeST RO7198457 (BNT122) 1L Melanoma with CPI Multiple ST (basket trial) Intratumoral Immunotherapy SAR441000 (BNT131) Solid tumors (IL-12sc, IL-15sushi, GM-CSF, IFNα) RiboMabs BNT141 Multiple ST BNT142 Multiple ST (CD3+CLDN6) RiboCytokines BNT151 Multiple ST (Optimized IL-2) BNT152/153 Multiple Solid Tumors (IL-7, IL-2) CAR-T Cells BNT211 Multiple ST (CLDN6) Next-Gen CP Immunomodulators BNT311 Multiple ST (PD-L1x4-1BB) BNT312 Multiple ST (CD40x4-1BB) Antibodies BNT321 Pancreatic Cancer (CA19-9) TLR7 Ligand BNT411 Multiple ST (TLR7) Infectious and Rare Diseases Influenza Up to 10 Infectious Disease Indications 5 Rare Disease Indications mRNA Others Phase 2/3 Phase 1/2 Phase 2 Phase 1 Start Phase 1 Start Phase 1 Start Phase 1 Report Phase 1 Phase 1/2 Report Phase 1/2 1We expect this topline data update to include an update on the ongoing study, including patient enrollment numbers, with full efficacy and safety data for an interim update expected in the second half of 2021; 2As the trial is sponsored and conducted by Sanofi, the timing of data updates is not under our control, and is subject to change by Sanofi. 3Our expectations for timing of milestones beyond 2020 are premised on and subject to the achievement of earlier milestones on their expected timelines. Press releases will be issued once first patient has been dosed. Start Phase 2 Start Phase 3 Start Phase 2 Start Phase 1 Report Phase 1/22 Trial progress update1 Data update Phase 1/2 Start Phase 1 Start Phase 1/2 Expected news flow Expected begin of trial Expected data readout / update Legend Data update Phase 1 Start first study Start first Phase 1 Start first Phase 1 Start Phase 1 Report Phase 1/2

Back-up

Year End Cash Balance 2019 (unaudited) – and Outlook Total Business In EURm December 31, December 31, 2019 2018 Cash and cash equivalents 520* 411 We expect net cash used in operating activities and other investments to total of approx. EUR 300m** in 2020. * EUR 520m -> approx. USD 584m **EUR 300m -> approx. USD 337m Conversion rate 1.12 The estimates above represent the most current information available to our management and do not present all necessary information for an understanding of our financial condition as of and the results of operations for the year ended December 31, 2019. We are currently preparing our financial results for the quarter and year ended December 31, 2019. There is no assurance that our cash and cash equivalents as of and for the year ended December 31, 2019 to be reported in our financial statements for the period, when finalized and reviewed, will not differ from the estimates provided. Any such differences could be material, and accordingly prospective investors should not place undue reliance on these estimates. The preliminary financial data included in this document has been prepared by, and is the responsibility of our management. Our independent registered public accounting firm has not audited, reviewed, compiled or applied agreed upon procedures with respect to the preliminary financial data. Financials update

. 2.1% 10.1% 10.3% Our RNA-LPX vaccine approach mRNA drug class | vaccine platforms Strong vaccine-induced ex vivo CD8 + T cell responses* across different cancer types FixVac iNeST HPV16-E7 Head Neck Cancer BNT113, HARE40 trial Mutant Neoantigen TNBC BNT114, TNBC Merit trial MAGE-A3 Melanoma BNT111, Lipomerit trial NY-ESO-1 Melanoma BNT111, Lipomerit trial 5.0%

Ribocytokines boost therapeutic efficacy of vaccination and PD-L1 blockade RiboCytokines Effect of tumor size on treatment success of vaccination + aPD-L1 Therapeutic efficacy of vaccination + aPD-L1 is reduced in large tumors CT26 tumor model, vaccine antigen:gp70 Complete rejection of large tumors when combining RNA vaccination, PD-L1 blockade as well as IL2 and IL7 Ribocytokines. CT26 tumor model, tumor size: 60mm2 CR: complete response, vaccine antigen:gp70 Ribocytokines boost the efficacy of vaccination + aPD-L1 in large tumors Vaccine + aPD-L1 +

© Copyright BioNTech SE 2019. All Rights Reserved. Jan 13, 2020 An der Goldgrube 12 55131 Mainz Germany T: +49 6131 9084-0 F: +49 6131 9084-390 M: info@biontech.de
